Scientists have created a massive DNA mapping system to study how DNA modifications evolve during aging through epigenetic changes.
Introduction
Scientists have launched an extensive mission to create the most detailed DNA aging map which shows epigenetic changes that occur with time. Scientists have created an extensive “giant map” known as the epigenetic atlas of aging which shows aging patterns across various human tissues while revealing which organs deteriorate quickly and why they do so. The increasing number of elderly people requires scientists to understand aging mechanisms at the molecular level.
The Epigenetic Landscape: Mapping DNA Methylation
DNA methylation serves as the fundamental research component because it modifies DNA sequences through cytosine base methylation which controls gene to gene expression changes that make tissues older and more prone to diseases. The aging process leads to specific expression by adding methyl groups to CpG sites.
DNA methylation precision deteriorates with age which leads patterns of CpG site methylation changes that follow predictable patterns.
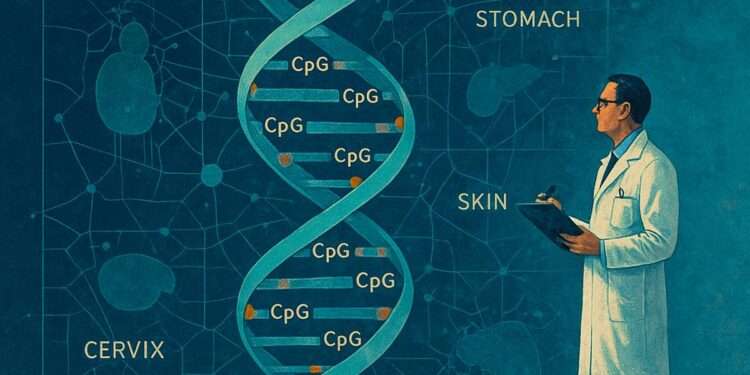
Scientists created an epigenetic aging atlas through DNA methylation pattern analysis of different human organs. The researchers discovered that the retina and stomach experience faster aging-related methylation changes than the cervix and skin tissues.
Scientists have built a massive DNA methylation atlas that shows how our genetic code changes with age. Read more on Scientific American .
The atlas revealed shared epigenetic markers which age at similar rates throughout different organs which could indicate shared biological aging mechanisms.
A Nature study presented a comprehensive age-related methylation map through the analysis of 17 human tissues across the entire adult life span. The research data helps scientists discover fresh molecular candidates for developing anti-aging treatments.
Epigenetic Clocks: Measuring Biological Age
The development of epigenetic clocks emerged from these atlases through the use of specific CpG site methylation levels to determine biological age.
The 2013 research by Steve Horvath established a multi-tissue epigenetic clock which uses 353 CpG markers to determine age in various tissues and cell types.
The Hannum clock used 71 blood-expressed CpG sites to generate highly accurate age predictions.
A recent Cambridge study links long-term air pollution exposure to higher dementia risk.
The GrimAge and PhenoAge models represent second-generation epigenetic clocks which combine health data with smoking records and inflammatory markers to calculate biological age through mortality risk assessment.
The DunedinPACE tool calculates aging speed instead of age to show how quickly or slowly someone ages compared to their expected rate.
The clocks enable multiple uses including disease risk assessment and mortality prediction and treatment effectiveness monitoring without needing extended time periods for results.
Tissue Aging Differentials and Anti-Aging Implications
The discovery of tissue-specific aging rates has brought about a major breakthrough in scientific understanding. The retina and stomach experience rapid methylation changes which makes them essential for studying age-related deterioration.
This knowledge:
The location of anti-aging therapy development becomes more specific through this discovery.
The method allows healthcare professionals to detect organ dysfunction at its early stages in organs that show vulnerability.
The method provides individualized aging progression forecasts through the analysis of specific tissues.
Future medical treatments involving targeted drugs and gene therapies could potentially reverse or decelerate tissue-specific aging through methylation signature reprogramming.
Beyond Methylation: Somatic Mutations and Epigenetic Instability
The epigenetic atlas depends mainly on methylation but aging produces multiple additional molecular processes.
The number of somatic mutations grows with time increases mainly in cells which experience high turnover rates and defective DNA repair mechanisms. The accumulation of genomic instability through these mutations leads to faster deterioration of cellular functions.
The aging process leads to increased variability in methylation patterns which shows distinct patterns between male and female populations. Research on elderly subjects confirmed that differentially methylated positions (DMPs) follow specific patterns.
The process of epigenetic drift which results in the progressive breakdown of coordinated methylation patterns becomes a defining feature of aging and contributes to cancer development and Alzheimer’s disease and cardiovascular diseases.
The changes that occur with time also depend on environmental factors and lifestyle choices and metabolic stress levels.
Toward Therapeutic Targets
Therapeutic research now has a solid foundation because scientists have discovered identical aging patterns in different body tissues.
The discovery of common epigenetic markers enables medical treatments to affect multiple body systems at once.
Scientists can develop specific organ-based treatments by studying how aging affects different tissues.
Experimental studies demonstrate that CRISPR-based epigenetic editing techniques successfully block essential CpG site methylation changes.
Scientists are working on developing epigenetic rejuvenation treatments which transform cells into younger states while preserving their original identity. Scientists have conducted initial mouse studies which demonstrate that partial cellular reprogramming techniques can both restore tissue functionality and correct methylation patterns.
Biological Age vs. Chronological Age: What’s the Difference?
The number of years we have lived since birth represents chronological age but biological age tracks our molecular and physiological wellness.
Epigenetic clocks measure the age difference between biological and chronological age by tracking how lifestyle choices and environmental factors and genetic makeup influence aging speed. The biological age of two individuals who share the same chronological age of 60 will differ significantly based on their smoking habits and their dietary choices and exercise routines and their health status.
The knowledge of this discrepancy enables people to receive age-specific medical care that matches their biological state instead of their actual age.
Global Implications: Medicine and Society
The discovery of an extensive epigenetic aging map generates multiple effects which extend past laboratory work.
Medical professionals will use basic blood tests to detect patients who face high risks of age-related diseases through methylation signature analysis before symptoms emerge.
The use of biological age data by governments enables them to monitor population wellness and create improved screening methods and resource distribution for areas with rapid biological aging.
The longevity industry has started to compete with other companies to create diagnostic tools and therapeutic solutions based on epigenetic clock technology for increasing human health duration and life expectancy.
The tools create ethical dilemmas because they raise questions about who should access biological age information including employers and insurers and governments. The protection of privacy protection remains essential to achieve the advantages that these tools provide.
Conclusion
The discovery of a massive multi-tissue epigenetic aging map represents a major breakthrough in human aging research. The DNA in our cells uses methylation patterns to record time’s passage through universal and tissue-specific sequences which span from the retina to the stomach and include all genes and chromosomes.
These findings:
The research reveals the mechanisms behind organ-specific aging rates.
Scientists can use epigenetic clock technology to develop new methods for biological age assessment.
The research provides essential knowledge for creating personalized medical treatments which aim to stop or reverse molecular aging processes.
The advancement of epigenetic mapping precision and editing technology will bring the possibility of specific anti-aging treatments into reach. Scientists can use the giant map to identify specific methylation sites for modification which could lead to increased human lifespan.